Our boat could become a giant battery
Doesn’t that sound cool? Well, it’s not really. At least not in the sense that I was thinking about. I am referring to what people call galvanic corrosion, which is not a good thing to have uncontrolled on a boat. Here is my layman’s understanding of this process and what to do about it.

When two dissimilar metals (ex. stainless steel and aluminum) are placed in contact with one another in an electrolyte solution (salt water for example) they can begin to react like a battery. When this occurs the less noble metal (aluminum in this case) begins to corrode. As I understand it, this process can be further accelerated with electrical wiring problems that leak stray current. More on that in a moment.
Why am I thinking about this now and what can be done about it? I mentioned a month ago that we were considering the addition of mast steps. We ultimately decided to install just two steps near the top of the mast to give a stable platform in case any maintenance work needs to be done up there. The steps we purchased are stainless steel. The bolts we will be using to attach the steps are also stainless. The mast however is aluminum, and as mentioned above, those two metals don’t really like to be placed in contact with one another. The solution in this case is to isolate the two metals from one another. There are a couple of products that people use for situations like this. Tef-Gel is one. We chose to get some Lanacote as it is a lot cheaper. I believe either should do the trick in this situation.
In other situations the galvanic reaction is controlled by using sacrificial anodes. For example, near the bottom of outboard engine there is a piece of metal bolted on, solely for the purpose of being eaten up by this galvanic reaction. The metal typically used for this is Zinc, one of the least noble of metals. I learned in our marine maintenance course that in fresh water we should really be using Magnesium instead of Zinc, and in brackish water we would use Aluminum. What do we actually have on our engines? I actually couldn’t tell you. I’ll have to check. So, the key with these sacrificial anodes, or Zincs as they are commonly referred to, is that they need to be checked and replaced on a regular basis. If they are doing there job they should, over time, be eaten away. Once they’re gone the metals which you don’t want to corrode would begin to suffer damage.
What about the stray current thing? I know much less about this so I’ll just quote wikipedia:

Yachts connected to improper grounded shore supply systems should employ a galvanic isolator to isolate from any stray currents in the marina (either from the shore mains or neighbouring yachts). Without the isolator, a galvanic corrosion path may be created damaging metal equipment below the waterline. Zinc anodes may help to prevent this, but often prove insufficient when the yacht stays in the marina for extended periods.
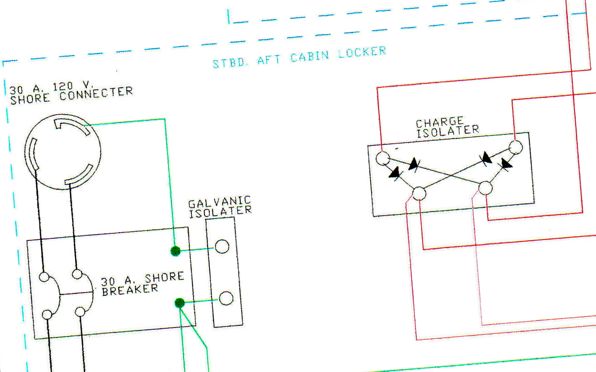
We do have a Galvanic Isolator installed on Katana and I assume that it is working, or at least I hope it is. Once we get out of marinas and away from shore power I don’t think we’ll have to be concerned with this issue much.


Another reason to like simple.
No shore power,we’re 12 volt all the time (sorry dear,no hair dryer,no microwave on this rig.)
And we can lower the mast with a brake winch to fix the stuff on top(although it’s an interesting maneuver on the water!)
I still have more than enough on my list of things to worry about!
The galvanic corrosion caused by shore power issues is still a bit of a mystery to me. I haven’t done much reading on it. Is there potentially still an issue if the boat beside you in the marina is hooked up to shore power and is having problems, even if you are not connected?
I think as long as you don’t have a completed circuit,it’s not an issue. If you don’t have a ground,you cant have a current flow.
In our case ,we can lift the motor out of the water and we have no metal below the waterline,eliminating the problem.
Zincs on our motor are 4 years old and look fine.
I’m sure others have more knowledge on this.
Just as important is the stray current that can zap you if you go in the water near boats hooked to shore power,and not properly grounded.
Makes sense, and I agree certainly about the electrocution thing. That would not be very cool!
It was recommended that we buy a little circuit tester (like the one linked below) that we plug into the shore power to see if they have wiring issues:
https://www.theelectricaltoolstore.com/index.php?main_page=product_info&products_id=186
I once reached from my Stiletto into the water to scoop some water to keep bait… and nearly dropped the pot. The handle got tingly. When I think of how many times I have gotten in the water at a marina to do some maintanance…. I still do, but I inspect the area and move very carefully.
The boat in the next slip had left with the shore power hooked up while he went fishing and it fell into the water. Normally this just burns up the head of the plug, but in this case one of the plugs had sheared off at the shore end, so electrons were pretty active in a ~ 20-foot circle.
Never, never, never leave the shore end in when you go for a day sail. there was a fatality a few years ago, a few towns up. I don’t know the details, but it involved children and a wiring fault a few boats away.
Our marina owner is very much on top of the whole power cord thing. We saw him get into a “discussion” with one of the boat owners on this subject the very first day we visited the place. Because of his influence we have learned to ensure that there is no way a live cord (our cord) can enter the water. That is not to say that every other boat owner is quite so conscientious, so caution is always justified.
Scariest to me is the idea that a thru hull could become part of that battery. We also have a galvanic isolator and we are putting capacitors on our RF ground when we connect it to the thru-hull even though there are other protections in that system.
Yes, the other boats in the marina can cause problems. It is a complicated puzzle to solve and easier to just assume the electrolysis problem is bad and keep your boat protected. You never know when you will wind up stuck in a “bad” marina somewhere in your travels. Good for you having a galvanic isolator.
Lifting the outboard engine helps; metal thru hulls can be problematic as can be metal rudder posts.
Besides the Lanicote, here is another cheep trick; on larger surfaces, use a panel from a plastic milk jug between the dissimilar surfaces. Many windlasses are installed on dissimilar backing plates resulting in frozen assemblies. This was especially a problem on some Lagoon 380s with Leroy Somer windlasses. Reassembly with the milk jug barrier reportedly resolved the problem.
Thanks for the tip on the milk jug, Kirk.
I think that our seacocks are “plastic” so with the engines raised, the only metal in the water should be the swim ladder if it’s down and the anchor/chain.
ABI, now SeaDog, I think, makes a folding aluminum mast step, which is what we used. http://www.greenboatstuff.com/abipoalfomas.html
I debated buying those but on the recommendation of the guy at the chandlery opted for the non-folding version.
http://bestmarineimports.com/ABI183223.jpg
His opinion was that the folding ones were great closer to the deck but higher up, the movement of the mast could cause them to pop open. The added security of having my foot surrounded was also a selling feature of the triangular ones.
Great writeup Mike!
I’ll bet that the fasteners holding those steps to the mast are not the only stainless on your mast… The stays and shrouds are stainless, aren’t they?
bob
Thanks Bob.
You’re definitely right about the other stainless. On the spring commissioning list that the previous owners gave us one of the items on it is to redo that corrosion blocker on all dissimilar metals.
It isn’t just the boat next to you in the marina that can cause a problem. It’s any boat in the marina. Water, especially salt or brackish water, is an excellent conductor of electricity. You also need to check for stray current before you go in the water for ANYTHING!!! If it tingles, get out, FAST! There are some older threads on the LA forum that discuss this issue. Just do a search.
No swimming in the marina for us!
It all boils down to this very wise piece of advice a friend who is a marine electrician told me:
Green is not a conductive color.
That simplifies things.